Monopar Announces Journal of Hepatology Publishes Physicians’ Letter to the Editor Demonstrating ALXN1840 Rapidly Improved Copper Balance in Wilson Disease Patients
WILMETTE, Ill., Sept. 23, 2025 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (“Monopar” or the “Company”) (Nasdaq: MNPR), a clinical-stage biopharmaceutical company developing innovative treatments for patients with unmet medical needs, today announced that the Journal of Hepatology has published a peer-reviewed Letter to the Editor (link), authored by leading Wilson disease physicians, entitled “Oral Bis-choline Tetrathiomolybdate Rapidly Improves Copper Balance in Patients with Wilson Disease.” Wilson disease is a rare and progressive genetic condition in which the body’s pathway for removing excess copper is compromised, leading to damage from toxic copper build-up in organs such as the liver and brain.
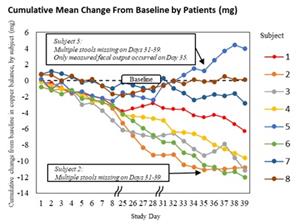
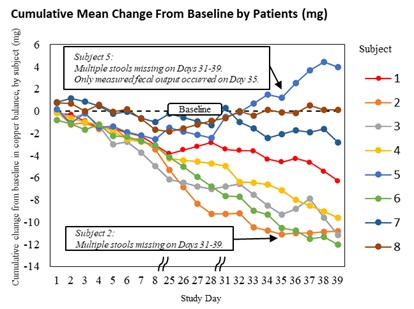
In the Letter to the Editor, the authors explain that the earlier conclusion from the Phase 2 ALXN1840-WD-204 study (NCT04573309) – that ALXN1840 did not promote copper excretion – was based on a methodological limitation in the copper balance equation, which only accounted for certain routes of copper loss. By controlling for the other routes of copper loss by comparing pre- and post-ALXN1840 treatment, the analysis demonstrates that ALXN1840 statistically significantly improved copper balance (increases copper excretion) over the duration of the study. Supplementary materials published alongside the Letter illustrate how the same primary copper balance data produced both the prior sponsor’s analysis as well as the results presented in the Letter.
Key findings on the effect of ALXN1840 on copper balance reported in the Letter:
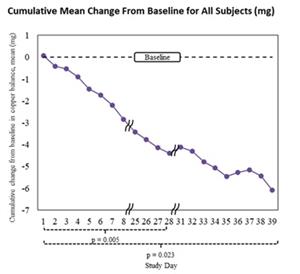
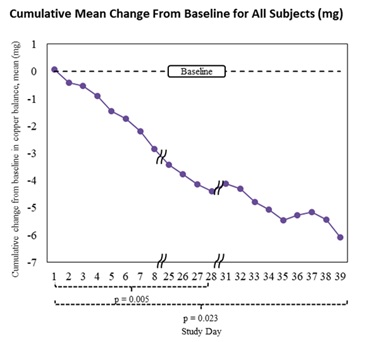
- 15 mg/day treatment period: mean daily difference -0.367 mg (p=0.005)
- Overall treatment period (includes patients with dose changes to 15 mg every other day and to 30 mg/day): mean daily difference -0.289 mg (p=0.023)
-
Cumulative mean change from baseline: -6.08 mg (95% CI: -10.18 mg to -1.98 mg), see graphs below
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage biopharmaceutical company with late-stage ALXN1840 for Wilson disease, and radiopharmaceutical programs including Phase 1-stage MNPR-101-Zr for imaging advanced cancers, and Phase 1a-stage MNPR-101-Lu and late preclinical-stage MNPR-101-Ac225 for the treatment of advanced cancers. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements involve risks and uncertainties including, but not limited to: uncertainties related to the regulatory process that Monopar intends to initiate related to ALXN1840 and the outcome thereof; the rate of market acceptance and competitiveness in terms of pricing, efficacy and safety, of any products for which Monopar receives marketing approval, and Monopar’s ability to competitively market any such products as compared to larger pharmaceutical firms; Monopar’s ability to raise sufficient funds in order for the Company to support continued preclinical, clinical, regulatory, precommercial and commercial development of its programs and to make contractual milestone payments, as well as its ability to further raise additional funds in the future to support any existing or future product candidate programs through completion of clinical trials, the approval processes and, if applicable, commercialization; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
Contact:
Monopar Therapeutics Inc.
Investor Relations
Quan Vu
Chief Financial Officer
vu@monopartx.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/9b7c7917-6cae-4321-bd40-82479a25b590
https://www.globenewswire.com/NewsRoom/AttachmentNg/38d44bf0-8b61-4cb7-b5a6-99b64ede71cb

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.